Stroke is a leading cause of death and disability and, currently, only acute ischemic stroke (AIS) can be treated within a limited treatment window of 4.5-6 hours after symptom onset. In contrast, no treatment for hemorrhagic stroke are generally approved even though blood pressure lowering treatment shows promising effects. For this reason, novel neuroprotective treatments of stroke are needed. One of these, the non-invasive treatment remote ischemic conditioning (RIC) conducted by short cycles of controlled reversible ischemia and reperfusion in a limb to attenuate prolonged ischemic reperfusion injury (IRI) in a remote organ, has shown promising results in preclinical studies and is being tested in large clinical studies. However, the protective signals in RIC have turned out to be elusive.
In the last decade, extracellular vesicles (EVs) have attracted increasing attention especially for their diagnostic biomarker potential in different diseases and as a readout to monitor treatment efficacy. In addition, these nanosized vesicles are released in the blood by a wide array of cell types and have the potential to function as long distance signaling systems carrying messages that can impact the function of the recipient cells. An interesting group of molecules with the potential of re-programming the receiving cell is the short non-coding microRNAs (miRNAs) that controls and modulates the translation of a large fraction of proteins. Changes in miRNAs will therefore have an impact on the expression profile of the cell and thereby their function.
We are studying changes of both EVs and miRNAs in stroke and as a consequence of RIC in both patient samples and in samples from healthy human volunteers. In addition, several of our studies are focused on elucidating the biological function of RIC induced EVs and miRNAs in stroke models.
Coordinator: Kim Ryun Drasbek
Studying remote ischemic conditioning miRNA effects on brain endothelial cells in in vitro stroke models of ischemia/reperfusion and inflammation
Acute ischemic stroke (AIS) is one of the leading causes of death and disabilities, and as such, it is of utmost importance to identify novel treatment options. Current acute treatments for AIS are limited to either thrombectomy or thrombolysis, both of which must be initiated within 4.5-6 hours of symptom onset. Remote ischemic conditioning (RIC) is a promising non-invasive treatment that is thought to activate the body's own protective mechanisms against damaging ischemia through circulating microRNAs (miRNAs).
To investigate this in further detail, we aim to characterize the cell reprogramming potential of four RIC associated miRNAs (RIC-miRNAs: miR-16-5p, miR-144-3p, miR-182-5p, and miR-451a) in a cellular stroke model to identify possible biological pathways responsible for protection against damaging ischemia in endothelial cells.
Pronounced transcriptional changes were present after RIC-miRNA transfection, with 149 unique downregulated and 212 upregulated differentially expressed genes in HBMECs after in vitro stroke mimicking the initial stages of AIS and RIC-miRNA transfection compared to all other conditions. These genes were involved in cell cycle regulation, DNA replication and pathways of energy metabolism. Thus, the selected RIC-miRNAs regulate pathways that may facilitate endothelial cell recovery and remodeling events from ischemic damage, offering new therapeutic avenues for AIS.
Ongoing studies: We are currently studying the biological function of the RIC-miRNAs in response to prolonged ischemia and inflammation in different in vitro stroke cell models.
Collaborators:
Katrine Tang Stenz, CFIN, Dept Clinical Medicine, AU
Jesper Just, Dept Molecular Medicine, AUH & CFIN, Dept Clinical Medicine, AU
Thomas Ravn Lassen, Dept Cardiology, AUH & Dept Clinical Medicine, AU
Hans Erik Bøtker, Dept Cardiology, AUH & Dept Clinical Medicine, AU
Kristian Vissing, Dept Public Health, AU
Xiu-Jie Wang, Dept Genetics and Developmental Biology, CAS, China
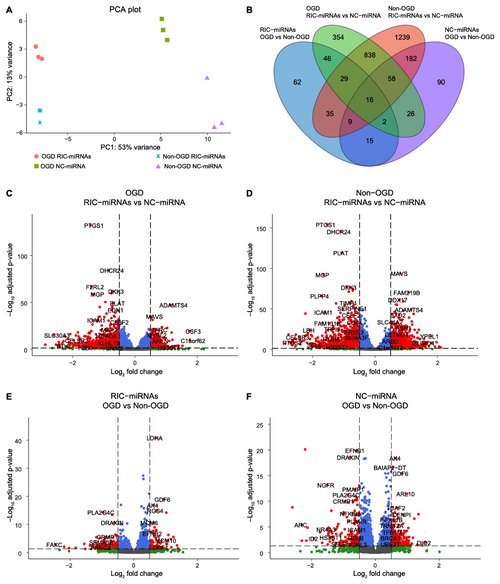
Figure legend:
Transfection with RIC-miRNAs changes gene expression profiles in HBMECs. A: Principal component analysis (PCA) visualizes the large separation between the groups where the transfection treatment creates a larger variance than oxygen tension. B: Venn diagram showing common differentially expressed genes (DEGs) in all of the analyses. C-F: Transfection with RIC-miRNAs gives rise to more DEGs than oxygen tension (OGD/non-OGD). Red dots: DEGs defined as a Log2FoldChange (Log2FC) > 0.5 and an adjusted p-value (padj) < ±0.05. Blue dots: padj < ±0.05, Log2FC < 0.5. Non-significant: Grey dots: padj > ±0.05, Log2FC < 0.5. Green dots: padj > ±0.05, Log2FC > 0.5. Total =14516 genes.
Extracellular vesicle surface markers and microRNA regulation in ENOS; a randomized-controlled-study of remote ischemic conditioning in acute ischemic stroke
Remote ischemic conditioning (RIC) has demonstrated neuroprotective effects, yet translating this into clinical efficacy has proven challenging. This study aims to uncover the molecular mechanisms underlying RIC’s protective effects in acute ischemic stroke (AIS) patients, which could potentially identify RIC specific biomarkers and new therapeutic targets.
We conducted the ENOS study, a patient-assessor blinded, sham-controlled clinical trial, to investigate the molecular changes in patients diagnosed with AIS. Patients were assigned to undergo either RIC, involving five cycles of transient ischemia and reperfusion of the arm, or a Sham treatment. We collected plasma samples at three time points: within 24 hours of hospital admission, two hours post-initial RIC, and after seven days, with RIC administered twice daily. Analysis focused on brain biomarkers, specifically extracellular vesicle (EV) surface markers and circulating microRNAs (miRNAs).
We identified significant differences in the regulation of CD62 on EVs and five specific miRNAs (miR-374a-5p, miR-20a-5p+miR-20b-5p, miR-19b-3p, miR-24-3p, miR-30d-5p) between the RIC and Sham groups. Notably, miR-374a-5p, known for its neuroprotective properties, emerged as a key mediator of the RIC response (p = 0.015). Furthermore, the changes of several of these miRNAs were correlated to improvements in red blood cell (RBC) deformability and aggregation during shear stress. In addition, we observed significant increases in CD62 on EVs at both the two-hour and seven-day follow-ups in the RIC group. These findings suggest that RIC induce specific changes in EV surface markers and circulating miRNAs that may be future biomarkers for the RIC response.
Collaborators:
Rebecca Best Jensen, CFIN, Dept Clinical Medicine, AU
Maria Kjølhede, Dept Neurology/Stroke Center, AUH & Dept Clinical Medicine, AU
Betina Elfving, TNU, Dept Clinical Medicine, AU
Jesper Just, Dept Molecular Medicine, AUH & CFIN, Dept Clinical Medicine, AU
Grethe Andersen, Dept Clinical Medicine, AU
Rolf Ankerlund Blauenfeldt, Dept Neurology/Stroke Center, AUH & Dept Clinical Medicine, AU
Malene Møller Jørgensen, Dept Clinical Immunology, AAUH & Dept Clinical Medicine, AAU
Rikke Bæk, Dept Clinical Immunology, AAUH
Lee-Ann Clegg, Dept Clinical Medicine, AAU
David C Hess, Dept Neurology, Augusta Uni, GA, USA
Figure legend:
Correlation analysis of significant miRNA measurements (hsa-miR-19b-3p, hsa-miR-30d-5p, hsa-miR-374a-5p, hsa-miR-20a/20b-5p, hsa-miR-24-3p), CD62/P-selectin, and clinical scores. The histograms on the diagonal show the distribution of each variable. The lower triangular panels depict scatter plots with fitted lines, illustrating the relationships between pairs of variables. The upper triangular panels display the correlation coefficients, with significance levels indicated by asterisks (*p<0.05, **p<0.01, ***p<0.001)
Ultra-early diagnosis of stroke, originating from either blocked or bleeding blood vessels in the brain, will save time and lead to faster treatment, which is of utmost importance in stroke. Unfortunately, current methods cannot distinguish between stroke types and a reliable diagnosis has to await imaging of the brain at the hospital delaying the start of treatment.
In the STIMULATE project, we will develop a blood based diagnostic test to distinguish between stroke types. When suffering a stroke, specific nano-particles are released from the stroke area into the blood. By combining characterization and quantification of these naturally occurring nano-particles with prehospital clinical assessments, we aim to develop a multiparametric diagnostic device for use in the ambulance that can help diagnose stroke type. A highly reliable diagnostic test will allow correct treatment of the stroke type to be started in the ambulance during transport to the hospital leading to better outcome.
We are currently evaluating extracellular vesicle biomarkers in 550 patients with different types of stroke as well as non-stroke patients with similar symptoms.
Collaborators:

Figure legend
A: Currently, no effective treatment can be initiated in the hyper-acute phase after stroke onset due to stroke patients presenting identical clinical symptoms even though they suffer from contrasting diseases such as acute ischemic stroke (AIS) and intracerebral hemorrhage (ICH). This results in infarct growth during transport until correct in-hospital diagnosis and treatment initiation. B: The development of a portable multiparametric device for ultra-early diagnostics in the ambulance will enable immediate treatment initiation prior to hospitalization. This will reduce infarct growth, thereby leading to a better outcome for the patients.
Exposing tissues to brief periods of ischemia confers resistance to subsequent prolonged ischemia (local ischemic preconditioning). Intriguingly, a systemic protective response can be induced by repeated brief ischemia of a limb (remote ischemic conditioning, RIC). A similar systemic protective effect seems achievable by traditional exercise regimens while ischemic resistance exercise training conducted as low-intensity blood flow restricted resistance exercise (BFRE) holds the potential to promote tissue rebuilding by repeated application. The protective processes of RIC and BFRE appear to be facilitated by miRNAs transported by secreted extracellular vesicles (EVs) from the site of occlusion to sites of organ damage. Clinically, RIC has been seen to reduce infarct size and/or improve outcomes in patients admitted with acute myocardial infarction or stroke.
The main goals of the project include:
Identification of the mechanisms by which RIC and BFRE evoke remote organ protection will, potentially, lead to future therapeutics using naturally secreted EVs as a drug delivery system in personalized treatment. This interdisciplinary program offers an ideal synergy to achieve the proposed objectives.
Collaborators:
Funding:
Figure legend:
Plasma extracellular vesicles following remote ischemic conditioning (RIC) accumulate in the infarcted hemisphere of a mouse stroke model, while RIC EVs are also taken up by human brain microvascular endothelial cells in culture. In addition, RIC EVs are protective when compared to pre-RIC EVs as well as EVs from a non-intervention control group of human volunteers, when analyzed in an in vitro model of stroke.